This training is intended for those charged with shipping samples to a diagnostic laboratory on behalf of Veterinary Services. The training does not cover shipping category A infectious substances or shipping on dry ice.
This training is valid through December 31, 2019
• Contacts: For questions about training content, please contact the Professional Development Staff: 301- 851-3545
• For help shipping to the National Veterinary Services Laboratories (NVSL), please contact NVSL Shipping: 515-337-7530
• For questions about conducting a foreign animal disease investigation, refer to Veterinary Services Guidance 12001.2, and the Foreign Animal Disease Investigation Manual (FAD PReP Manual) and contact the Area Veterinarian in Charge (AVIC) and National Preparedness and Incident Coordination (NPIC) staff.
• This training is intended for those charged with shipping samples to a diagnostic laboratory on behalf of Veterinary Services. The training does not cover shipping category A infectious substances or shipping on dry ice.
• No prior experience with shipping infectious substances is required or assumed.
• This training is valid through December 31, 2019
• Learning Outcomes: Upon completion of this training, you will be able to package, label, and ship potential Category B infectious substances to a diagnostic laboratory for VS program activities.
• Note: there is no audio for this training.
• Please note that this presentation opens in a separate window from the Shipping Course USDAtraining.com site.
• At any time, you may navigate to others sections within the Shipping Course, by returning to the Shipping Course page within USDAtraining.com and using the Navigation pane (on the left side of your screen)
• At any time, you can click on the Glossary (within Shipping Course Resources in your USDAtraining.com Navigation pane) to download definitions of terms used within this presentation.
• There is a knowledge review at the end of the course. The review is not graded, but enables you to measure your comprehension of the course material.
• A printable version of this transcript is available within the Shipping Course Resources on USDAtraining.com
1. Regulations, definitions, and training requirements
2. Process: how to classify, identify, select packaging, and mark, label & document for shipment
3. The Bottom Line: shipper’s responsibilities, best practices, penalties, list of packaging manufacturers and distributors
4. Knowledge review, shipping with FedEx, references, and conclusion
• Hazardous Materials Regulations (49 CFR; US Department of Transportation)
• Dangerous Goods Regulations (IATA)
Hazardous Materials Regulations (HMR; 49 CFR Parts 100-185)
• Issued by the U.S. Department of Transportation (US DOT)
• Establish requirements governing the commercial transportation of hazmat by highway, rail, vessel and air
• Link: http://www.phmsa.dot.gov/hazmat/regs (opens in new window)
Hazardous Materials Regulations (HMR)
• Laboratory specimens are often considered hazardous materials by the US DOT
• Once you have collected specimens, you are responsible for complying with regulations for:
Dangerous Goods Regulations (DGR): Airlines – IATA
• Major air carriers (including FedEx and UPS) are members of the International Air Transport Association (IATA)
• IATA develops and publishes regulations in the IATA Dangerous Goods Regulations (DGR)
• The IATA DGR comply with international flight regulations promulgated by the United Nations
• Domestic flights are subject to each country’s civil aviation authority. In the United States, the Department of Transportation and the Federal Aviation Administration regulate the transportation of hazardous materials.
• Link to the IATA web site: http://www.iata.org/publications/dgr/pages/index.aspx (opens in new window)
• The following pages contain definitions for terms used throughout the course
• Biological substance: description of an infectious substance, Category B
• Category A: an infectious substance transported in a form that, when exposure occurs, is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals
• Category B: an infectious substance which does not meet the criteria for inclusion in Category A. A category B infectious substance is not in a form generally capable of causing permanent disability or life- threatening or fatal disease in otherwise healthy humans or animals
• Cultures: result from a process by which pathogens are intentionally propagated
• Exempt patient specimen: direct patient specimen for which there is minimal likelihood that pathogens are present. Exempt from regulations if properly packaged and marked
• Hazard classes: hazardous materials are divided into nine hazard classes, depending on the type of danger they pose
• Hazardous material (dangerous goods): substance or material that poses a risk to health, safety, property, or the environment when transported in commerce
• Hazardous materials regulations (HMR): 49 CFR Parts 171 - 180. The HMR classify clinical specimens and biological substances in class 6, division 2 as either Category A or Category B
• Infectious substance: a material known or reasonably expected to contain pathogens (agents that can cause disease in humans or animals)
• Patient specimen: collected directly from humans or animals, including but not limited to excreta, secreta, blood, tissues and tissue fluid, swabs
• Proper shipping name: a standard name used to identify the article or substance on the outside of the package and on the airbill
• Shipping paper: a shipping document that accompanies a package. May also be referred to as an airbill, air waybill, or shipping document, depending on the regulatory body or shipping company.
• UN number: every proper shipping name is assigned an identification number by the United Nations (UN) Committee of Experts
• Unregulated samples: specimens which are not expected to contain infectious agents; examples of unregulated samples include dried blood spots for genetic testing and samples where any pathogens present have been neutralized or inactivated
• Depend on the type of material being shipped
• This presentation covers shipping Category B substances (49 CFR 173.199), exempt patient specimens, and unregulated samples
• Regulations require that personnel involved in packaging and shipping of infectious substances have appropriate training
• Training requirements vary depending on category of infectious substances that are being shipped
• Each person who offers or transports a Category B infectious substance must know about the requirements of 49 CFR 173.199 (opens in new window)
• This presentation is intended for persons offering Category B substances, exempt patient specimens, and unregulated samples for transport
• Shipping Category B substances does not require certification
• This training does not cover shipping Category A substances or shipping with dry ice. If you suspect you have a Category A substance, or are not certain of the category, contact your Area Veterinarian in Charge (AVIC).
As a shipper, you are responsible for:
• Classification
• Identification
• Packaging
• Marking and Labeling
• Documentation
• Refrigerants
• Any additional legal obligations (permits)
1. Classification: Classify the sample (based on whether it contains infectious material or not)
2. Identification: Determine how to identify the sample
3. Packaging: Obtain proper packaging materials and follow packing instructions
4. Marking, Labeling, and Documentation: Prepare the package for shipping
1. Classification
• Shippers must classify substances to determine whether materials are dangerous goods or not
• Unregulated (non-regulated) samples are not subject to the requirements as Division 6.2 material:
• There are nine hazard classes. Other hazard classes such as toxins may be encountered in VS disease programs
• See the Classification Flow Chart for guidance on how to classify samples
• Division 6.2 substances are divided into 2 categories
• Category A:
• Category B: any substance that does not meet the definition of Category A; moderately hazardous and/or potentially pathogenic
• Some examples of materials that would be included in Category B are:
• Most specimens sent to NVSL (Ames or Plum Island) are considered to be infectious substances (either Category A or Category B)
• Link to Diagnostic Testing at NVSL
• Ship as an exempt animal specimen when:
• One example of exempt animal specimens:
• Most specimens sent to NVSL (Ames or Plum Island) are considered to be infectious substances (either Category A or Category B)
• Link to Diagnostic Testing at NVSL
Which of the following examples would be an exempt animal specimen?
A. BSE surveillance samples
B. Routine slaughter surveillance samples
C. FAD investigation samples
D. Tuberculosis samples
Correct Answer B: Routine surveillance samples would be an exempt animal specimen. The rest are all Category B.
2. Identification: assign a proper shipping name
• Proper shipping names are standardized to avoid confusion
• Each proper shipping name corresponds with a standard UN number
• Biological substance, Category B (UN 3373)
• Note: Exempt animal specimens must be marked as such on the package, but are not assigned a proper shipping name or UN number, as they are not found on the list of dangerous goods
• Division 6.2 Infectious substances are defined as “substances known or reasonably expected to contain pathogens”
• IATA requires that Infectious Substances be classified in Division 6.2 and assigned the appropriate UN codes:
• All dangerous goods must be assigned only one proper shipping name and corresponding UN number, which identifies the dangerous goods on the package and shipping paperwork
• Exempt specimens are not assigned proper shipping names or UN numbers, but the term “Exempt animal specimen” must appear on the package
• One example of exempt animal specimens:
Which of the following is not assigned a proper shipping name or UN number?
A. Category B
B. Category A, affecting humans
C. Exempt animal specimen
D. Category A, affecting animals
Correct Answer B: Exempt animal specimens are not assigned a proper shipping name or UN number because they are not listed as a dangerous good. The words 'exempt animal specimen' must still appear on the package.
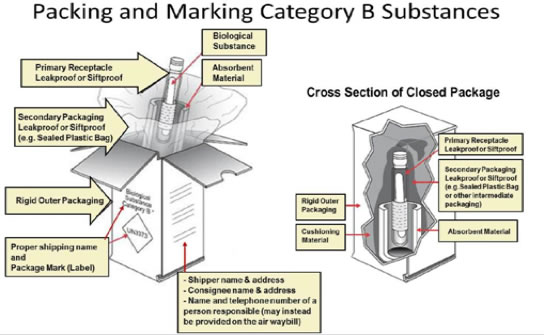
3. Packaging
• Select proper packaging
• Packages have 3 basic components:
• This section covers packaging for Category B, Biological Substances
• Preparation for shipping Category A, Infectious Substances requires additional training!
• The packaging for both Category B and exempt patient specimens consists of three basic components:
• Leakage or loss of contents from packages containing unregulated biological materials will likely be perceived and handled as hazardous materials by commercial carriers. Liability for losses and damage related to these leaking packages will most likely fall on the shipper.
• Category B is an infectious substance which does not meet the criteria for inclusion in Category A
• Suspected Category B materials must be assigned to UN 3373
• The proper shipping name of UN 3373 is Biological Substance, Category B
• See Packing Instruction 650 (from IATA; for shipment by air) for details
• Other dangerous goods must not be packed in the same packaging as Division 6.2 Infectious Substances unless they are necessary for maintaining the viability, stabilizing, or preventing degradation or neutralizing the hazards of the infectious substances
• Adequate absorbent material must be used for liquid shipments
• Solid shipments:
• Packages require orientation arrows only if a primary receptacle exceeds 50 ml
• For fresh tissues and swabs, place a frozen ice pack (not dry ice, not ice cubes) next to the samples. Ice packs are not needed for formalin-fixed samples
• Minimal likelihood that pathogens are present are present in samples
• Must be properly packaged, including absorbent materials for liquid specimens
• Mark as exempt animal specimen
• If the patient specimen contains or is reasonably expected to contain Category A or B pathogens, then it cannot be packaged as an exempt patient specimen
• If in doubt, contact destination lab for guidance
Which of the following components must be used to package both Category B and exempt patient specimens (select all that apply):
A. Leak-proof primary receptacle (specimen container)
B. Leak-proof secondary packaging
C. Absorbent material if liquid sample
D. Outer packaging
Correct Answer: All of the above
4. Marking, Labeling, and Documentation
• Marking: “Exempt animal specimen” – or – “Biological substance, Category B” and UN 3373 mark
• Itemized list of contents (between secondary and outer packagings)
• Airbill or shipping document
• The proper shipping name, Biological Substance, Category B must be marked in letters at least 6 mm high adjacent to the UN 3373 mark
• Samples inactivated by fixation in 10% formalin do not require the UN3373 mark
• The name and address of both the shipper and the consignee must be on the package
• The name and telephone number of a person who is either:
• Must be included on a written document (such as an air waybill or bill of lading) or on the outer packaging
• An itemized list of contents (e.g. VS 10-4 or BSE submission form) must be enclosed between the secondary and outer packagings
Federal employees obtain packaging materials from NVSL
BSE (and CSF) Fresh Tissue Kit (comes in 5, 10, and 25 tube sizes):
• Conical tubes (primary receptacle)
• Barcode labels
• Ice pack
• Absorbent material
• Leak-proof plastic bag (secondary packaging)
• Submission forms
• Styrofoam box
• UN 3373 label
• Rigid outer packaging
• Packing and shipping instructions
CSF (classical swine fever) Kit:
Use the BSE (CSF) Fresh
Small diagnostic kit (use for FAD investigations):
Note: Primary receptacles are NOT included
• Ice pack
• Absorbent material
• Leak-proof plastic bag (secondary packaging)
• Submission form
• Styrofoam box
• UN 3373 label
• Rigid outer packaging
• Packing and shipping instructions
Large diagnostic kit (use for FAD investigations):
Note: Primary receptacles are NOT included
• Ice packs
• Absorbent material
• Leak-proof plastic bag (secondary packaging)
• Submission forms
• Styrofoam box
• UN 3373 label
• Rigid outer packaging
• Packing and shipping instructions
TB kit, slaughter:
• 6x6 zip lock bag for ear tag
• Borate buffer jar and formalin jar (primary receptacles)
• Absorbent material
• Leak-proof plastic bag (secondary packaging)
• Submission forms
• Rigid outer packaging with required markings
• Packing and shipping instructions
• Return label
TB kit, deer:
• Formalin jar and whirl-pak bag (primary receptacles)
• Absorbent material
• Ice pack
• Return label
• Leak-proof plastic bag (secondary packaging)
• Submission forms
• Rigid outer packaging with required markings
• Packing and shipping instructions


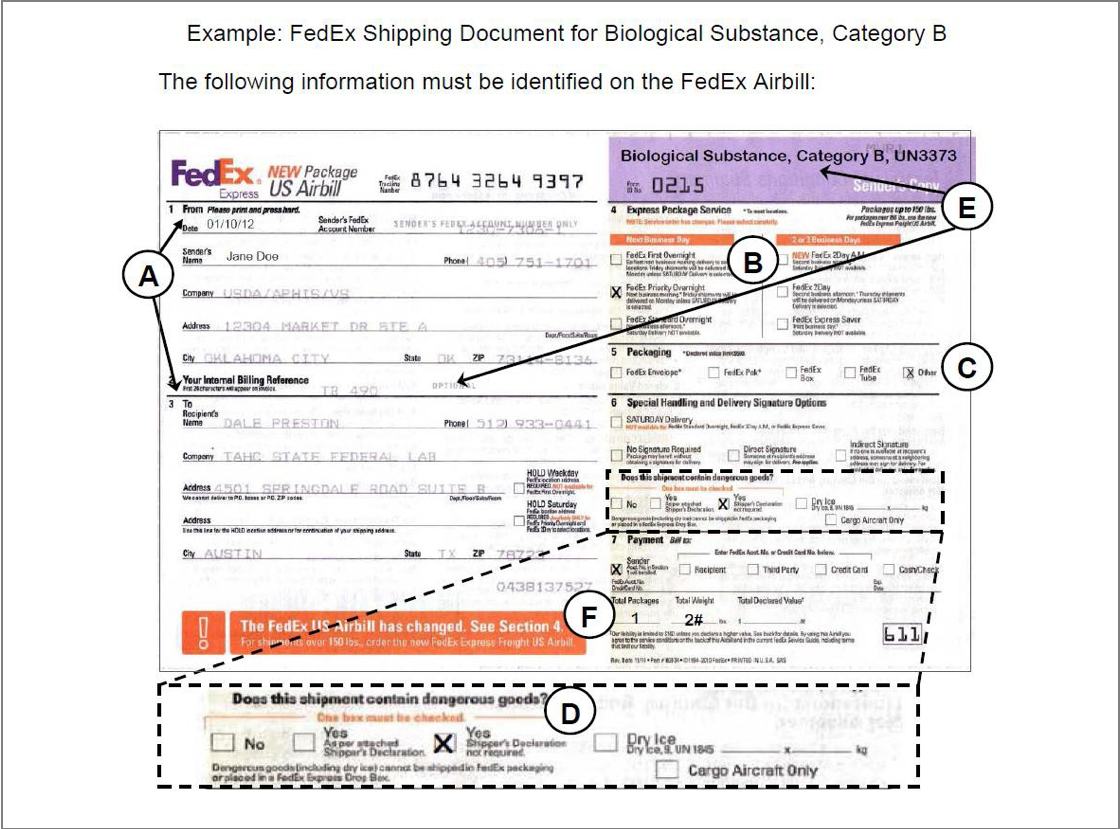
(Written description of video content provided on next slide)
Page 61 Packaging Video Transcript
This video demonstrates how to properly package category B specimens using an NVSL Shipping Box.
The following are links to information you may want to save or print: